Blood pressure medication recalled due to chemicals' possible link to cancer

FILE IMAGE - A nurse measures a patient’s blood pressure in a file image dated May 7, 2021. (Photo by Christoph Soeder/picture alliance via Getty Images)
Two lots of quinapril and hydrochlorothiazide tablets have been recalled due to the presence of nitrosamines, according to a notice posted on the Food and Drug Administration's website.
Nitrosamines are common in water and foods, including cured and grilled meats, dairy products, and vegetables, and may increase the risk of cancer if people are exposed to them above acceptable levels over long periods of time.
The pink, round tablets contain 20 milligrams of quinapril and 12.5 milligrams of hydrochlorothiazide and are supplied in 90-count bottles with an expiration date of January 2023. The drug is used to treat hypertension and lower blood pressure.
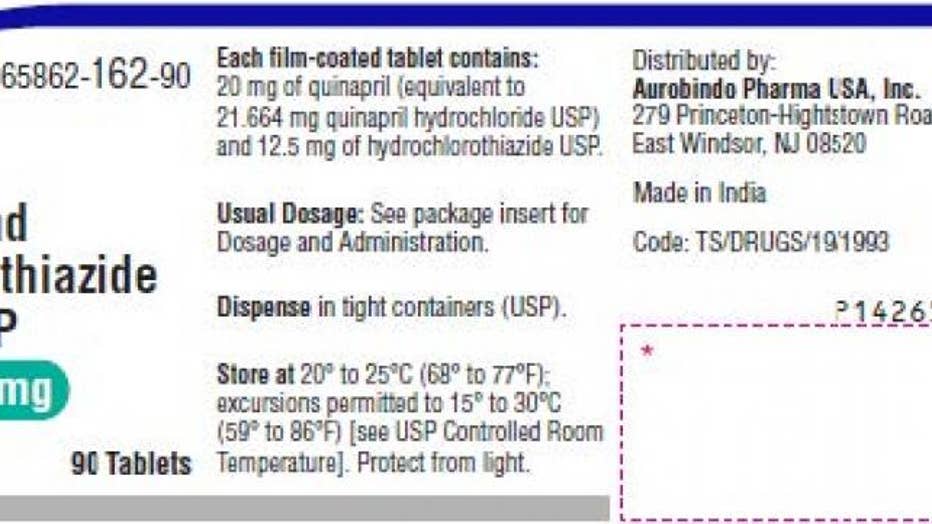
Two lots of quinapril and hydrochlorothiazide tablets have been recalled due to the presence of nitrosamines, according a notice posted on the Food and Drug Administration's website.
East Windsor, New Jersey-based Aurobindo Pharma USA Inc. shipped the recalled batches, known as QE2021005-A and QE2021010-A, to customers nationwide in May 2021. To date, the company has not received any reports of adverse events related to the recall.
KIA RECALLS 72,000 SPORTAGE MODELS, SAYS PARK OUTSIDE DUE TO ENGINE FIRE RISK
Aurobindo is working with the firm Qualanex to arrange for the return of the recalled lots. Qualanex will notify its distributors and customers by phone and in writing to immediately discontinue distribution of the recalled lots.
"Patients should contact their doctor or health care provider about whether to continue taking their medication or whether to consider an alternative treatment prior to returning their medication," the FDA advisory states.
Consumers should also contact their physicians or health care providers if they have experienced any problems that may be related to taking or using the drug.
Medical questions can be directed to Aurobindo at 1-866-850-2876 or by email at pvg@aurobindousa.com. Questions about the return of the recalled product can be addressed to Qualanex at 1-888-504-2014.
Adverse reactions or quality problems experienced with the use of the drug may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax at 1-800-FDA-0178.


